Kohlrausch's law of indendent migration of ions states that the molar conductance of infinite dilution (limiting molar conductivity ) is the sum of the individual contributions of the anions and cations of the electrolyte.
It can be given in the mathematical form as:
λ0M = v+λ0+ + v-λ0-
λ0+ and λ0- are the limiting molar conductivity of cation and anion respectively.
For NaCl,
λ0M = λ0Na+ + λ0Cl-
And for Al2(SO4)3,
λ0 Al2(SO4)3 =2 λ0Al3+ + λ0SO4 2-
chemistry assignment help, Mercury halides, Potassium dichromate, Bio polymers and Biodegradable polymers, Carbon fiber, Chemicals in food, Compounds of xenon, Copper sulphate penta hydrate, Copper sulphate penta hydrate, Corrosion, Energy producing cells, Group 18 elements, Imperfections in solids, Indicators in acid - base titration, Isomerisms, Lyophilic colloids and lyophobic colloids, Noble gases, extraction of aluminium, chemical equations, Refining of metals, Silver halides,
Phosphine gas (PH3)
In the laboratory phosphine is prepared by heating white phosphorus with concentrated caustic alkali solution in an inert atmosphere of oil gas or CO2.
P4 + 3NaOH + 3H2O ------------> 3NaH2PO2 (Sodium hypophosphite) + PH3 (phosphine)
Metal phosphides on hydrolysis form phosphine
Ca3P2 + 6H2O ------------> 3Ca(OH)2 + 2PH3
A pure sample of phosphine can also be prepared by heating phosphorous acid
4H3PO3 ---------------> 3H3PO4 + PH3
Phosphine has pyramidal structure and is a weaker base than NH3
P4 + 3NaOH + 3H2O ------------> 3NaH2PO2 (Sodium hypophosphite) + PH3 (phosphine)
Metal phosphides on hydrolysis form phosphine
Ca3P2 + 6H2O ------------> 3Ca(OH)2 + 2PH3
A pure sample of phosphine can also be prepared by heating phosphorous acid
4H3PO3 ---------------> 3H3PO4 + PH3
Phosphine has pyramidal structure and is a weaker base than NH3
Labels:
p-block element,
phosphine,
phosphorus
Oxides Of Phosphorus
The main oxides of phosphorus are phosphorus trioxide (P4O6) and phosphorus pentoxide (P4O10). Phosphorous trioxide is regarded as the anhydride of phosphorous acid (H3PO4). phosphorus trioxide (P4O6) is prepared by heating phosphorous in limited supply of oxygen. P4O10 is prepared by burning white phosphorous in excess of air or oxygen.
P4 + 3O2 -------------> P4O6
P4 + 5 O2 ------------> P4O10
P4O10 has great affinity for water and hence it is used as a dehydrating agent. It can dehydrate HNO3 and H2SO4 to yield N2O5 and SO3 respectively.
2H2SO4 + P4O10 ------------> 2SO3 + 4HPO3
P4O6 and P4O10 dissolves in water to give phosphorus acid and orthophosphoric acid respectively.
P4O6 + 6H2O -------------> 4H3PO3
P4O10 + 6H2O --------------> 4H3PO4
Related article oxyacids of phosphorus
P4 + 3O2 -------------> P4O6
P4 + 5 O2 ------------> P4O10
P4O10 has great affinity for water and hence it is used as a dehydrating agent. It can dehydrate HNO3 and H2SO4 to yield N2O5 and SO3 respectively.
2H2SO4 + P4O10 ------------> 2SO3 + 4HPO3
P4O6 and P4O10 dissolves in water to give phosphorus acid and orthophosphoric acid respectively.
P4O6 + 6H2O -------------> 4H3PO3
P4O10 + 6H2O --------------> 4H3PO4
Related article oxyacids of phosphorus
Extraction of sulphur
Sulphur occurs in nature in the elemental forms, as metal sulphides and as sulphates. Sulphur also occurs as H2S present in natural gas.
Sulphur is extracted by the following methods
1. Frasch process
In this process, sulphur is extracted from underground deposits by pumping super heated steam down the beds to melt the sulphur and then blown out the molten sulphur with compressed air.
2. Extraction of sulphur from natural gas
Natural gas contains a large amount of hydrogen sulphides (H2S). Hydrogen sulphide is first absorbed in monomethanolamine and then converts H2S into sulphur by the following reactions.
2H2S + 3O2 ------------> 2SO2 + 2H2O
2H2S + SO2 ------------> 3/8 S8 + 2H2O (at 673K and Fe2O3 as catalyst)
Related article extraction of aluminium
extraction of copper
Sulphur is extracted by the following methods
1. Frasch process
In this process, sulphur is extracted from underground deposits by pumping super heated steam down the beds to melt the sulphur and then blown out the molten sulphur with compressed air.
2. Extraction of sulphur from natural gas
Natural gas contains a large amount of hydrogen sulphides (H2S). Hydrogen sulphide is first absorbed in monomethanolamine and then converts H2S into sulphur by the following reactions.
2H2S + 3O2 ------------> 2SO2 + 2H2O
2H2S + SO2 ------------> 3/8 S8 + 2H2O (at 673K and Fe2O3 as catalyst)
Related article extraction of aluminium
extraction of copper
Oxides of Xenon ( XeO3 and XeO4)
Xenon trioxide (XeO3)
XeO3 prepared by the slow hydrolysis of XeF6
XeF6 + 3H2O ------------> XeO3 + 6HF
Xenon trioxide is soluble in water and its aqueous solution is weakly acidic.
XeO3 + H2O <--------> H+ + HXeO4 – Xenate ion
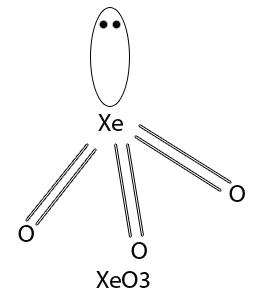
XeO3 has pyramidal structrure in which Xe is in sp3 hybridisation.
Xenon tetroxide (XeO4)
It is prepared by treating barium perxenate (Ba2XeO6) with anhydrous sulphuric acid.
Ba2XeO6 + 2H2SO4 -----------> XeO4 + 2BaSO4 + 2H2O
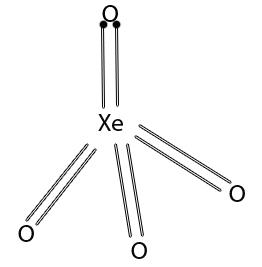
Xenon tetroxide is highly unstable and has tetrahedral structure.
Related article fluorides of xenon
For more detail visit indiastudychannel.com
XeO3 prepared by the slow hydrolysis of XeF6
XeF6 + 3H2O ------------> XeO3 + 6HF
Xenon trioxide is soluble in water and its aqueous solution is weakly acidic.
XeO3 + H2O <--------> H+ + HXeO4 – Xenate ion
XeO3 has pyramidal structrure in which Xe is in sp3 hybridisation.

Xenon tetroxide (XeO4)
It is prepared by treating barium perxenate (Ba2XeO6) with anhydrous sulphuric acid.
Ba2XeO6 + 2H2SO4 -----------> XeO4 + 2BaSO4 + 2H2O
Xenon tetroxide is highly unstable and has tetrahedral structure.

Related article fluorides of xenon
For more detail visit indiastudychannel.com
Hydrogen sulphide (H2S)
Hydrogen sulphide is prepared in laboratory by the action of dilute HCl or dilute H2SO4 on ferrous sulphide in Kipp’s apparatus.
FeS + H2SO4 ---------->4 + H2S
Physical properties
It is colourless gas with the smell of rotten eggs. It is denser than air and is soluble in water.
Chemical properties
1. Combustibility
Hydrogen sulphide burns in limited supply of oxygen to form sulphur. In presence of excess of oxygen, it gives sulphur dioxide.
2. Acidic property
H2S is a weak dibasic acid and forms two types of salts namely bisulphides and sulphides
NaOH + H2S ----------> NaHS + H2O
2NaOH + H2S ----------> Na2S + 2H2O
3. Action with metals
When H2S is passed over hot metals the sulphides and hydrogen are formed
Cu + H2S -----------> CuS + H2
4. Hydrogen sulphide in qualitative analysis
H2S precipitates metal sulphides having characteristic colours from metal salt solutions in acidic or alkaline medium. Therefore H2S is used in qualitative analysis to identify metal ions belonging to different classes.
In acidic solution, it precipitates group 2 cations (Hg2+, Cu2+, Pb2+, Bi3+, Sb2+, Sn2+, As3+, Cd2+ and Sn4+) as their sulphides with characteristic (Co2+, Ni2+,Zn2+ and Mn2+) as their sulphides with characteristic colours.
Related article Sulphur
FeS + H2SO4 ---------->4 + H2S
Physical properties
It is colourless gas with the smell of rotten eggs. It is denser than air and is soluble in water.
Chemical properties
1. Combustibility
Hydrogen sulphide burns in limited supply of oxygen to form sulphur. In presence of excess of oxygen, it gives sulphur dioxide.
2. Acidic property
H2S is a weak dibasic acid and forms two types of salts namely bisulphides and sulphides
NaOH + H2S ----------> NaHS + H2O
2NaOH + H2S ----------> Na2S + 2H2O
3. Action with metals
When H2S is passed over hot metals the sulphides and hydrogen are formed
Cu + H2S -----------> CuS + H2
4. Hydrogen sulphide in qualitative analysis
H2S precipitates metal sulphides having characteristic colours from metal salt solutions in acidic or alkaline medium. Therefore H2S is used in qualitative analysis to identify metal ions belonging to different classes.
In acidic solution, it precipitates group 2 cations (Hg2+, Cu2+, Pb2+, Bi3+, Sb2+, Sn2+, As3+, Cd2+ and Sn4+) as their sulphides with characteristic (Co2+, Ni2+,Zn2+ and Mn2+) as their sulphides with characteristic colours.
Related article Sulphur
Subscribe to:
Posts (Atom)