


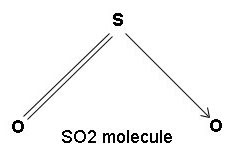
 Related article sulphur dioxide
Related article sulphur dioxide
chemistry assignment help, Mercury halides, Potassium dichromate, Bio polymers and Biodegradable polymers, Carbon fiber, Chemicals in food, Compounds of xenon, Copper sulphate penta hydrate, Copper sulphate penta hydrate, Corrosion, Energy producing cells, Group 18 elements, Imperfections in solids, Indicators in acid - base titration, Isomerisms, Lyophilic colloids and lyophobic colloids, Noble gases, extraction of aluminium, chemical equations, Refining of metals, Silver halides,